Yes According to the United States EPA performance definitions, the Xenex UV light product cannot: “Disinfect Rooms”, “High Level Disinfect Rooms”, or “Decontaminate Rooms”. Also, Xenex cannot market their UV devices as a “Sporicidal process” and they cannot claim to make room surfaces safe from spore contamination. Xenex is deceiving and scamming the public with claims that are completely untrue. Their deception must stop.
Documentation: https://altapure.com/the-uv-light-deception/
Press Release: https://altapure.com/xenex-uv-light-fails-hospital-spore-test/
No. The various UV light products on the market do not “Disinfect”, “Sterilize”, or “Decontaminate” rooms, and they do not meet the United States EPA performance criteria to make such claims under United States Environmental Protection Agency (EPA) regulation documents:
Product Performance Test Guidelines OCSPP 810.2200: Disinfectants
Product Performance Test Guidelines: OCSPP 810.2100: Sterilants
Yes. Drs. Raga and C. Donskey both obtained at least a 6 Log reduction of a covid-19 like challenge.
Link: Research and Data
Link: N95 Mask Page
Coronaviruses are proven to live for 5 to 28 days on stainless steel surfaces, but were also found to live on various surfaces in a maritime environment for at least 17 days.
To learn more about Coronaviruses and how long they can live on surfaces click here.
To learn more about Log Reduction or Log Kill click here.
No. Altapure recommends the use of an EPA-approved PAA disinfectant that degrades into harmless end products.
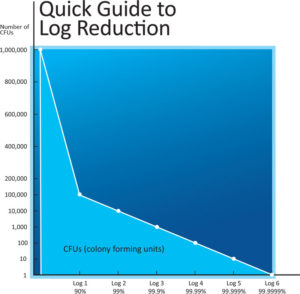
It is important to understand what Log Reduction is and why it is important to the process of surface disinfection, surface sterilization, and surface decontamination. Scientists, engineers, and other professionals who are responsible, or even legally responsible, for preventing illness and contamination, are concerned with Log Reduction or elimination of pathogenic bio-burden.
The term Log is short for logarithm, a mathematical term for a power to which a number can be raised. For example, using 10 as the given number, a Log 2 increase can be shown as 10^2 or 10 x 10 = 100.
Alternatively, a Log Reduction is taking the power in the opposite direction. For example, a Log Reduction of 1.0 Log is equivalent to a 10 fold reduction or, stated another way, moving down one decimal place, or a 90% reduction.
Product efficacy testing is done by counting the number of "colony forming units" (CFU) of the given pathogen / bacteria at the start of the treatment, and then performing a count again after the required treatment time. The result of the difference between the start and end numbers is then expressed as a Log Reduction.
For example, if the number of bacteria or bacterial colony forming units (CFU) in the beginning was one million or 1,000,000 (or 10^6), and the end result after the treatment was 1,000 (or 10^3) survivors, that would be a 3.0 Log Reduction (Log 3 reduction) or a reduction of 99.9%.
As a rule of thumb, for every additional Log Reduction number, you add the number 9 to the percentage reduction, so a Log Reduction of 3.0 Log is a 99.9% reduction compared with a Log Reduction of 6.0 Log which is equivalent to a 99.9999% reduction.
Below is an example of Log Reduction values using a starting point of one (1) million bacteria or 1,000,000 CFU's on a surface (ie: under bed rails in a hospital), as outlined below:
Log Reduction Number of cfu's Percent Reduction
0 log (Log 0) 1,000,000 0%
1 log (Log 1) 100,000 90%
2 log (Log 2) 10,000 99%
3 log (Log 3) 1,000 99.9%
4 log (Log 4) 100 99.99%
5 log (Log 5) 10 99.999%
6 log (Log 6) 1 99.9999%
WHY UNDERSTANDING LOG REDUCTION IS IMPORTANT:
Hospital surfaces can be contaminated with pathogenic organisms (bio-burden), and only achieving a Log Reduction below 6.0 Log means dangerous viruses, bacteria, fungus, and Clostridium difficile (C-diff) spores, can or will be left behind to proliferate and repopulate surfaces within the treated area. The literature has shown that bio-burden can be spread around to contaminate patients and/or grow new bacterial and fungal colonies on new surfaces. (1)
The number of bacterial survivors is very important because they can quickly increase their populations exponentially / logarithmically. For example, Staphylococcus aureus or (S. aureus) (under ideal conditions) doubles in 24-30 minutes (Generation Time, G), this means 1,000 or 10^3 or Log 3, bacterial survivors would increase to 2,000 after 30 minutes, after 60 minutes they would increase to 4,000, and after two hours to 16,000 and then increase to over one million or 1,024,000 after 5 hours or more, if the growing environment is optimal.
No. Altapure ® has pushed the envelope of aerosol generation technology, and offers a paradigm change with respect to large area high-level disinfection.
Altapure Offers:
Fastest Treatment Time A process that can treat a standard patient room in less than 50 minutes entry to exit, and where the treated area can be immediately used.
Full Efficacy A process that offers a 6 Log kill and no growth for: Bacteria, Viruses, Spores, and Fungus, for all exposed surfaces in the treated area. * See studies by Dr. C. Donskey, D. Maki, W. Rutala Ph.D.
Dry & Residue Free A “dry” process that quickly evaporates and leaves no residue.
Smallest Aerosol – Aerosol fog particles are generated in the sub-micron range with an average particle size of 0.69 micron, offering real gas-like performance and advantages.
Complete Coverage A dense gas-like aerosol cloud that fills large complex areas, long runs, and multiple areas, using a single machine.
Uniqueness An aerosol that is dry to the touch, offers gas like performance, but also offers the advantages of liquid contact.
Extremely Low Chemical Content – An aerosol that has only 0.88 % Hydrogen Peroxide, and 0.18 % Peracetic Acid.
Process Validation – Entire treatment process can be easily verified with biological indicators indicating highest efficacy level in industry– full spore kill for G. stearothermophilus spores.
Yes. The Altapure technology using an EPA approved PAA disinfectant will consistently deliver a 6 Log + kill, including spores like Clostridium difficile (C. diff).
For more information, please see this page: Test Results & Efficacy
The AP-4 ™ system is an advanced ultrasonic product capable of delivering a dense cloud of sub-micron droplets for the high-level disinfection of large spaces such as those found in a typical hospital.
The deployed sub-micron aerosol delivers gas-like performance and large, “3-D area coverage ™ ”, that can fill and treat multiple connected areas, complex geometries, long horizontal and vertical runs, and high-level disinfect or decontaminate the various surfaces within these spaces, including any equipment or objects therein.
Less than fifty (50) minutes.
A standard 3,000 cubic foot hospital room can be completely disinfected and repopulated in less than 45 minutes. This includes the time for setup and breakdown of the system, which takes a total of 10 minutes. Our high level disinfection process consists of 4 stages:
* Deploy - in the deploy stage the AP-4 ™ fills all areas of the room with the sub-micron aerosolized PAA agent.
* Dwell - the PAA solution is suspended in the air as a sub-micron sized aerosol, bombarding and destroying the pathogens through oxidation.
* Dehumidification - will remove the PAA aerosol from the room through our Air Reprocessor system housed in the AP-4 ™.
* Deodorization - the Air Reprocessor scrubs the air with an Activated Charcoal and HEPA filtration system to remove any remaining odor.
For more information, please see this page: Process Steps
Altapure's clinically proven process will deliver a predictable and consistent log 6+ reduction (99.9999% kill) when used in accordance with our instructions. More importantly, we achieve a “no growth” result for Viruses * Bacteria * Spores, in the treated space, including under tables, around corners, and in attached spaces like patient bathrooms.
When competitors publish their data, you must be careful to determine exactly what bacteria or virus the log reduction data is for, and where the biological challenges were located in the room, especially outside line of sight from the treatment device. More importantly, it is important to determine what spores (hardest to kill) were used in the testing. Were the spores located in challenging locations in the rooms and connected spaces?
It is important to note that third party laboratories, medical researchers, and hospitals, have proved that the Altapure technology can achieve “'No Growth” on all tested surfaces in the treated space, for bacterial spores like Clostridium difficile (C. diff / CDF / C-diff) and even hearty "gold standard" test organisms like G. Stearothermophilus spores (the same organism used daily in every hospital as a biological control test for all steam sterilizers).
For more information, please see this page: Test Results & Efficacy
No waiting / Immediate Access. Once the treatment cycle has finished, the treated room is safe to enter and is available for immediate occupation.
For more information, please see this page: Aerosol Liquid & Process Safety
If contaminated equipment, personnel, or devices, are brought into a room that has been treated by Altapure, it is possible to introduce and pass this new contamination to surfaces in that room, or even to any patient(s) in that room and infect them. This is why strict control protocols needs to be put into place and followed.
It is important to remember that peer reviewed clinical research has shown, according to Rutala et al. (2010), that patients hospitalized in rooms previously occupied by individuals infected or colonized with Methicillin-resistant Staphylococcus aureus (MRSA), [58] Vancomycin-resistant Enterococcus (VRE), [59] or Clostridium difficile (C. diff / CDF / C-diff) [60] are at significant risk of acquiring these organisms from contaminated environmental surfaces. [62] For example, Shaughnesy et al. (2011) stated that room assignment has been shown to be important in the acquisition of hospital-acquired infections, with a 40% increased risk of acquiring Methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin-resistant enterococcus (VRE) infection when the previous room occupant was positive. [27] [29] This is why it is important to clean each patient room before reoccupying it with a new patient.
Altapure's technology and process provides the certainty and confidence, that the patient is entering an extremely clean hospital room or operating space, that was treated with a methodology that offers a “Total Kill” and “No Growth Standard” for: Viruses, Bacteria, and Spores.
For more information, please see this page: Test Results & Efficacy
It is actually important for all high level disinfection equipment in the industry to meet this standard, since IEC60601-1 has stringent requirements for “conducted emissions” and it is essential that equipment meets this standard, given its close proximity to patients and other equipment in the area that may be used for patient care. If large area disinfection equipment does not meet this standard, it could possibly disrupt other hospital equipment connected to the same electrical system, and cause that equipment to malfunction. This electrical standard is very important.
Yes. The AP-4 ™ is remotely operated using a small, hand held controller that is touch screen activated. It communicates via wireless means with one or more AP-4 ™ machines and any ancillary devices. The remote control device is approved for use in all medial, and scientific environments. It meets all FCC regulations and our transceiver is approved for use in Europe and Japan.
No. We have done extensive testing on computers, electronics, televisions, and other equipment commonly found in hospital rooms and ICU units, with no corrosion issues. Methodist Hospital in Indianapolis, IN performed 1,300 deploys in the Critical Care areas with no reported equipment problems. A Level 1 Trauma Center in the Northeast, that averaged 5,000 deploys per year using Altapure's HJ-30i system, has also reported no evidence of corrosion damage.
For more information, please see this page: Aerosol Liquid & Process Safety
About 15 rooms.
No. However, it’s not a pleasant experience.
For more information, please see this page: Aerosol Liquid & Process Safety
Yes.
While Altapure has not yet had the opportunity to conduct testing against the Ebola virus, it has achieved “total kill” / “no growth” against the Poliovirus (VR-1562), per the AOAC International's test methods ( Please see this page: Test Results & Efficacy ).
The CDC recommends using a U.S. Environmental Protection Agency (EPA) registered hospital disinfectant with a label claim for a non-enveloped virus (ie: Norovirus, Rotavirus, Adenovirus, Poliovirus) to disinfect environmental surfaces in rooms of patients with suspected or confirmed Ebola virus infection.
The CDC also states that EPA-registered hospital disinfectants with label claims against non-enveloped viruses (ie: Poliovirus) are broadly antiviral and capable of inactivating both enveloped and non-enveloped viruses. However, as a precaution, selection of a disinfectant product with a higher potency than what is normally required for an enveloped virus, is being recommended at this time.
It is important to remember that all large area, high-level disinfection systems, should be used as an adjunct to regular cleaning / disinfection procedures for hard room surfaces. All federal labeling and directions for use must be followed.
Please visit the following CDC link for more information:
http://www.cdc.gov/vhf/ebola/hcp/environmental-infection-control-in-hospitals.html
Altapure meets the recommended level of performance, and it is believed that Altapure's technology can achieve a more consistent, faster, and better result, than any other large area, high level disinfection option currently available to the world today.
After testing various chemical products, Altapure chose to work with the PAA / Peroxyacetic Acid chemistry contained for the following reasons:
a) EPA approved for fogging.
b) Delivers a rapid kill for viruses, bacteria, and spores.
c) Decomposes and dries into safe and environmentally friendly components of: water vapor, oxygen, and acetic acid (vinegar) vapor.
d) Leaves no residue once dry.
e) Can obtain full spore kill with only 0.88 % H2O2 & 0.18 % PAA.
f) Pharmaceutical quality raw ingredients.
g) For over 20 years, PAA chemistry has been used in hospitals and clinics.
* Click here to learn more about Altapure and the AP-4 ™.