Real Science-Based Solutions
Altapure is helping to answer the call for real, science-based solutions to the supply shortage of N95 Masks due to recent 'pandemics' and worldwide illness.
Reminder: A 6 Log reduction is necessary according to the United States Environmental Protection Agency (EPA) to claim disinfection, sterilization, and sporicidal properties.
For More Information
about what Log Reduction or Log Kill means...
Summary - Pathogen High-Level Disinfection Performance
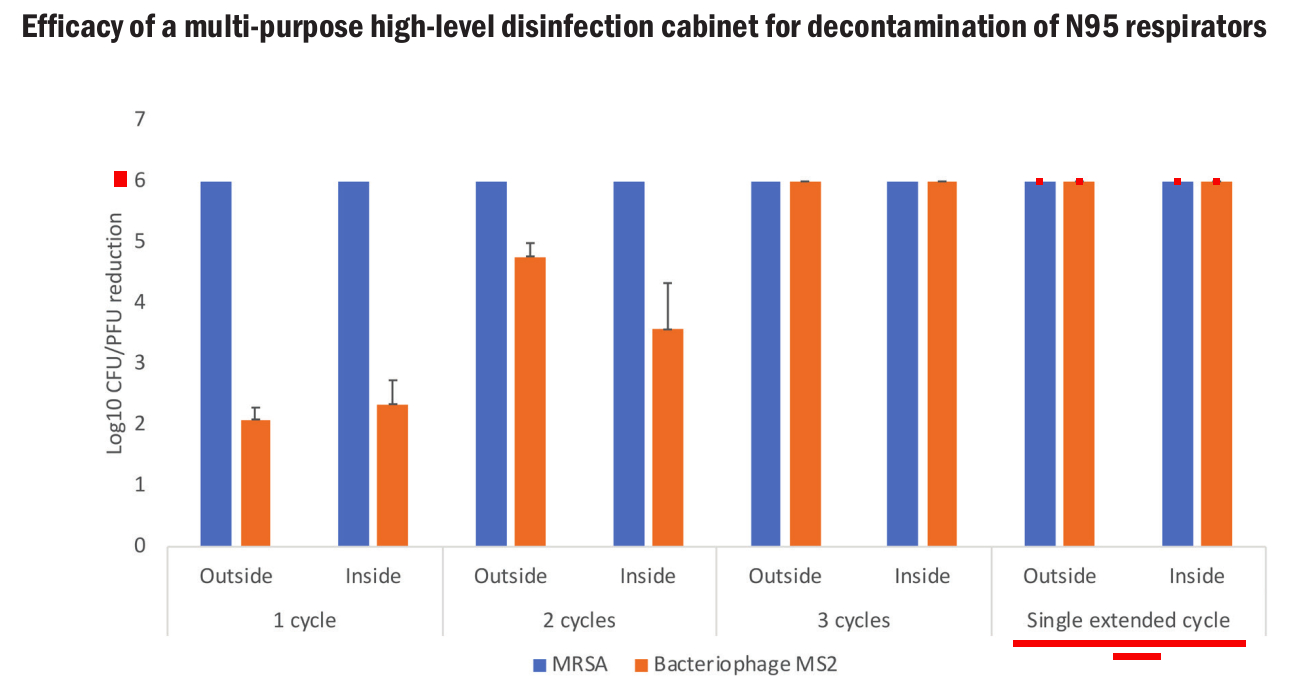
The data from the two (2) independent peer reviewed studies shown below, show Altapure’s performance when treating N95 masks either in Altapure’s disinfection cabinet or in a treatment room. Their data shows:
Coronavirus - Obtained a >6 log reduction for N95 mask filter materials contaminated with coronavirus 2 (SARS-CoV-2) that causes COVID-19 (due to surrogate kill data below).
Bacteriophage MS2 - Obtained a >6 log reduction for N95 mask filter materials contaminated with a 6 log reduction for the bacteriophage MS2, which is a surrogate for coronavirus 2 (SARS-CoV-2).
MRSA - Obtained a >6 log reduction for N95 mask filter materials contaminated with methicillin-resistant Staphylococcus aureus (MRSA).
C. difficile spores - Obtained a >6 log reduction for N95 mask filter materials contaminated with C. difficile spores, on N95 mask filter materials.
G. stearothermophilus spores - Obtained a 6 log reduction for N95 mask filter materials contaminated with G. stearothermophilus Spores.
Process Cycle Time Comparisons For N95 Masks
Advantages Of Using Altapure For N95 Mask and PPE Disinfection & Decontamination
a) Onsite Treatment - Can rapidly disinfect and decontaminate N95 masks and PPE at healthcare facilities.
b) Results & Safety – Enhanced +6 Log reduction for Corona virus on N95 masks increases the safety and confidence level of the treatment outcome, and meets the EPA’s highest performance criteria.
c) Speed - Rapid turnover of thousands of disinfected N95 masks in only 76 minutes.
d) No Shared N95 Masks – Healthcare staff can quickly get their personal form fitted masks and gear back after disinfection.
e) Volume - Can safely disinfect at least 2,000 N95 respirators in a (2447 cu. ft) volume room.
f) Scalability – This process is scalable and can be set up in real-world hospital settings.
g) Non-toxic – Peracetic Acid does not linger on surfaces and it rapidly biodegrades into its base elements of hydrogen peroxide and acetic acid (vinager), which then quickly fall apart into water, oxygen and carbon dioxide.
h) Vapor Free – No detectable off-gassing from the treated masks after the disinfection cycle.
i) Full Viral Disinfection - Log 6 reduction for coronavirus 2 (SARS-CoV-2) on N95 mask surfaces far exceeds the EPA’s requirements of only 3 Log reduction, significantly increasing viricidal safety.
j) Full Pathogen Disinfection - Log 6 reduction for methicillin-resistant Staphylococcus aureus (MRSA), C. difficile spores, and G. stearothermophilus spores, on N95 mask surfaces, meeting the EPA’s highest performance standards.
k) Dual Hospital Use - The AP-4 HLDS system can also be repurposed for terminal disinfection of patient rooms when it is not being used to disinfect N95 masks and PPE.
l) Cellulose Safe - Compatible for use with cellulose-containing masks and PPE materials, unlike vaporized hydrogen peroxide (VHP).
Independent Studies
Independent Study # 02
N95 Mask Treatment - Large Treatment Room
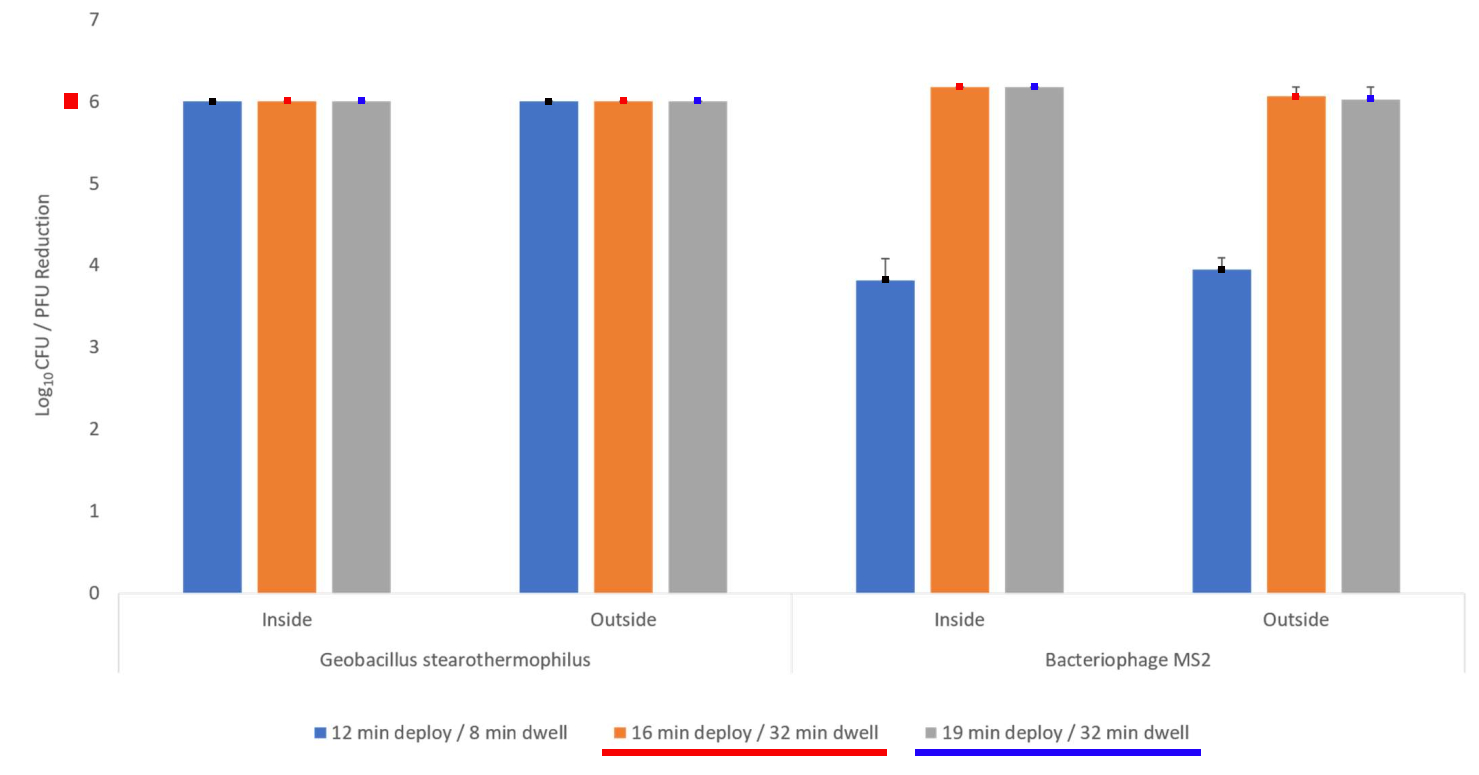
Altapure's AP-4 ™ high-level disinfection system (HLDS ™) was located in the center of a 2,447 cubic foot sealed room, and decontaminated all of the N95 masks located and hanging inside that room, and achieved full disinfection for all of these N95 masks with a 76 minute cycle.
This process for the complete treatment of N95 masks located within the treated room, met the criteria for disinfection and decontamination of the N95 masks, achieving >6-log 10 reductions on N-95 respirator filter material with a 76-minute process cycle, for:
- Bacteriophage MS2 (Coronavirus surrogate)
- G. stearothermophilus spores




Citations:
(104) Raju S., Amrita, J., et al., “Scalable In-hospital Decontamination of N95 Filtering Facepiece Respirator with a Peracetic Acid Room Disinfection System”.