EXPOSING THE UV-C / PX-UV MYTH & CONSUMER DECEPTION
I. Introduction
The following is a recent example of a UV-C light product not meeting “disinfection” performance expectations at a Veterans Administration (VA) hospital in Ohio:
“The number of C-Diff rooms has increased, despite current sanitation procedures. The Louis Stokes Cleveland VA Medical Center currently utilizes the Tru-D Smart UVC Part Number: 0367AOLF, but we are still not getting the desired results and the level of disinfection expected to especially hard to reach areas.”
(1) - Louis Stokes VA Hospital, Cleveland, OH, 2017, FedBizOpps Solicitation Number: VA250-17-Q-0774.
The UV-C and PX-UV light room treatment industry is not regulated by any United States Government Agency. Sellers of UV-C and PX-UV light room treatment products have and are, committing consumer deception by falsely claiming that their products can “disinfect”, “sterilize”, or “decontaminate”, when clearly they cannot as shown by the new research data further below.
The industry's assertions have been debunked by numerous independent peer-reviewed research papers, reported in key research journals, showing that UV room treatment systems do not meet the minimum Federal Government performance standards for Disinfection, Hospital Disinfection, and Sterilization. Even unsupported claims of Decontamination, are extremely serious and can impact the life, health, and safety, of the public as these claims are being relied by medical professionals to prevent injury and death.
It matters not whether the UV-C or PX-UV light is produced by Xenex, Tru-D or Clorox, they are all hampered by the same laws of physics and limitations, such as:
- Diminishing power over increasing distance
- Angle of the exposed surfaces
- Surface shadowing
Despite slick advertising and purchased studies, the fact remains, a “totally clean” or “totally disinfected” room cannot be achieved by using UV room treatment products. Failure to “disinfect” surfaces and leaving a viable pathogenic bio-burden that can infect others is not acceptable.
II. What Is Disinfection? And How Is the UV-C & PX-UV Industry Committing Deception?
In general, in order to claim disinfection a cleaning process must attain at least a 6 Log reduction of specific organisms, in a specified period of time. Sterilization means a complete kill of at least 6+ Log test material leaving no growth on any treated surfaces.
There are different United States Government standards for claiming surface Disinfection and Sterilization. The following is very brief summary – most are time dependent:
a) “General Disinfection” = 6 Log reduction of “Staphylococcus aureus” AND “Salmonella enterica”
b) “Hospital Disinfection” = 6 Log reduction of “Staphylococcus aureus” AND “Pseudomonas aeruginosa”
c) “Disinfectant with Fungicidal claims” = 6 Log reduction of “Trichophyton mentagrophytes”
d) “Sterilant with C-Diff. Spore Claims” = 6 Log reduction of “Clostridium difficile (C. difficile) spores”
See further below: OCSPP 810.2200 (3) (2), OCSPP 810.2200 (5) & (6) (2), OCSPP 810.2200 (9)(e) (2), and OCSPPP 810.2100 (d)(2) and (g) (3).
The UV light room treatment industry should NOT be claiming the above performance standards unless their product(s) can meet or exceed each specific requirement. Deceptive advertising occurs when a claim is made, but where the product cannot actually meet the requirement(s).
The data shown further below demonstrates that both UV-C and PX-UV cannot meet these EPA standards.
III. Understanding Log Reduction Is Essential To Eliminating Pathogenic Risk
Hospital surfaces can be contaminated with many pathogenic bio-burden, and only achieving a Log Reduction at or below 6.0 Log means dangerous viruses, bacteria, fungi, and C. difficile (C-diff) spores, can or will be left behind to proliferate and repopulate surfaces within the treated room. The literature has shown that bio-burden can be spread around to contaminate patients and/or grow new bacterial and fungal colonies on new surfaces. (14)
The number of bacterial survivors is very important because they can quickly increase their populations exponentially / logarithmically. For example, Staphylococcus aureus or (S. aureus) (under ideal conditions) doubles in 24-30 minutes (Generation Time, G), so this means 1,000 or 10^3 or Log 3, bacterial survivors would increase to 2,000 after 30 minutes, after 60 minutes they would increase to 4,000, and after two hours to 16,000 and then increase to over one million or 1,024,000 after 5 hours or more, if the growing environment is optimal.
IV. Examples of the Deception
Here are just a few examples from sellers of UV-C and PX-UV light room treatment products committing consumer deception by falsely claiming that their products can “disinfect” when clearly, they cannot.
Example # 1 – Xenex
"https://www.xenex.com/about-xenex"
“In use in more hospitals than any other UV disinfection device, Xenex offers the only Pulsed Xenon UV disinfection system on the market. Xenex Germ-Zapping Robots® are developed and designed to be highly effective, efficient and portable, allowing for the proven and systematic disinfection of any space within a healthcare facility." (emphasis added) (11)
Example # 2 - Tru-D
"http://tru-d.com/benefits/"
“Only Tru-D provides guaranteed, total room disinfection and has been validated by nearly all existing independent research on UVC room disinfection technology. As health care-associated infections continue to be a major threat to hospital reimbursements and the bottom line, hospital leaders must be diligent in choosing which technologies they invest in to help combat this serious problem. Proven consistent outcomes provide a baseline of disinfection that can only be accomplished with Tru-D’s method of UVC dose measurement." (emphasis added) (12)
Example # 3 - Surfacide
"http://www.surfacide.com/"
“The Surfacide Helios system implements multiple emitters that allows us to disinfect all areas of the healthcare environments in a single cycle including the bathroom." (13)
“With Surfacide’s three emitters operating during the same disinfection cycle, no exposed surface is left untouched." (emphasis added) (13)
Why UV Room Disinfection Fails:
Distance
Shadowing
Surface Angle to Light
V. UV light room treatment systems do NOT meet the above definitions as evidenced by the independent peer-reviewed research papers discussed below:
1) Michelle Nerandzic, and Curtis Donskey, MD et al.
"Evaluation Of An Automated Ultraviolet Radiation Device For Decontamination of Clostridium difficile and Other Healthcare-associated Pathogens In Hospital Rooms", BioMedCentral, BMC Infectious Diseases, 2010, 10:197 . (8)
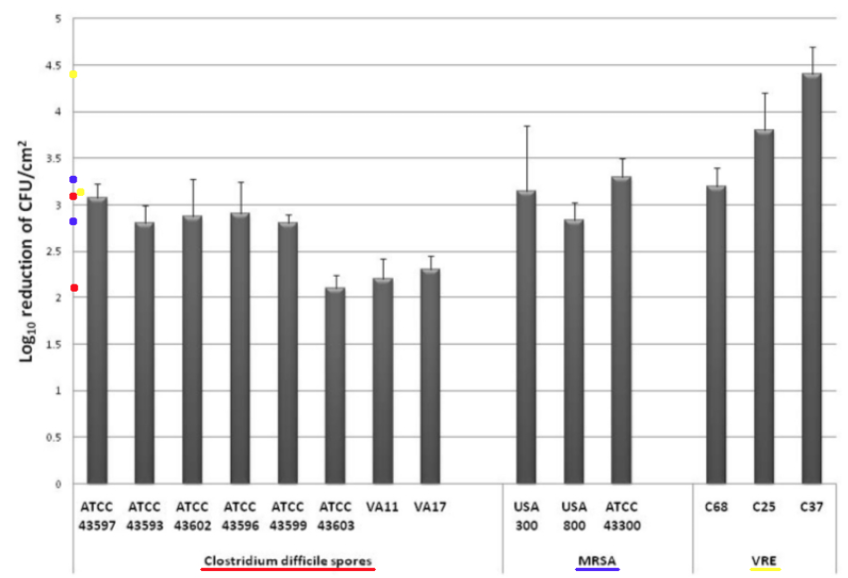

Comments – Figure 2: The C. difficile spore data in Figure 2 above shows a Log Reduction range of (2.2 to 3.1) for direct UV-C light exposure for 45 minutes.
Per Federal standards, if a test surface is contaminated with 1,000,000 bacteria, and a Log Reduction of about 2.2 Log to 3.1 Log is obtained for C. difficile spores by exposure to direct UV-C light, that means there will still be between about less than 1,000 to almost 10,000 C. difficile spore survivors remaining. This is NOT disinfection, decontamination, or sterilization, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
The MRSA (Staphylococcus aureus) data in Figure 2 above shows a Log Reduction range of (2.8 to 3.4) for direct UV-C light exposure.
If a test surface is contaminated with 1,000,000 bacteria, per Federal standards, and a Log Reduction of about 2.8 Log to 3.4 Log is shown for MRSA by exposure to direct UV-C light, that means there will still be between about more than 100 to more than 1,000 MRSA survivors remaining that can exponentially increase their population and constitute a health risk. This is NOT disinfection, decontamination, or sterilization, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
Conclusion: This study reinforces the currently reported research data that UV-C room treatment systems, like Tru-D, do NOT meet the legal definitions for disinfection, hospital disinfection, sterilization, or as a sporicidal against C. difficle, per the United States EPA and Federal regulations. (2)(3)
2) Jennifer L. Cadnum, and Curtis Donskey, MD, et al.:
"Effect of Variation in Test methods on Performance of Ultraviolet-C Radiation Room Decontamination", Infection Control & Hospital Epidemiology, November 2016. (6)
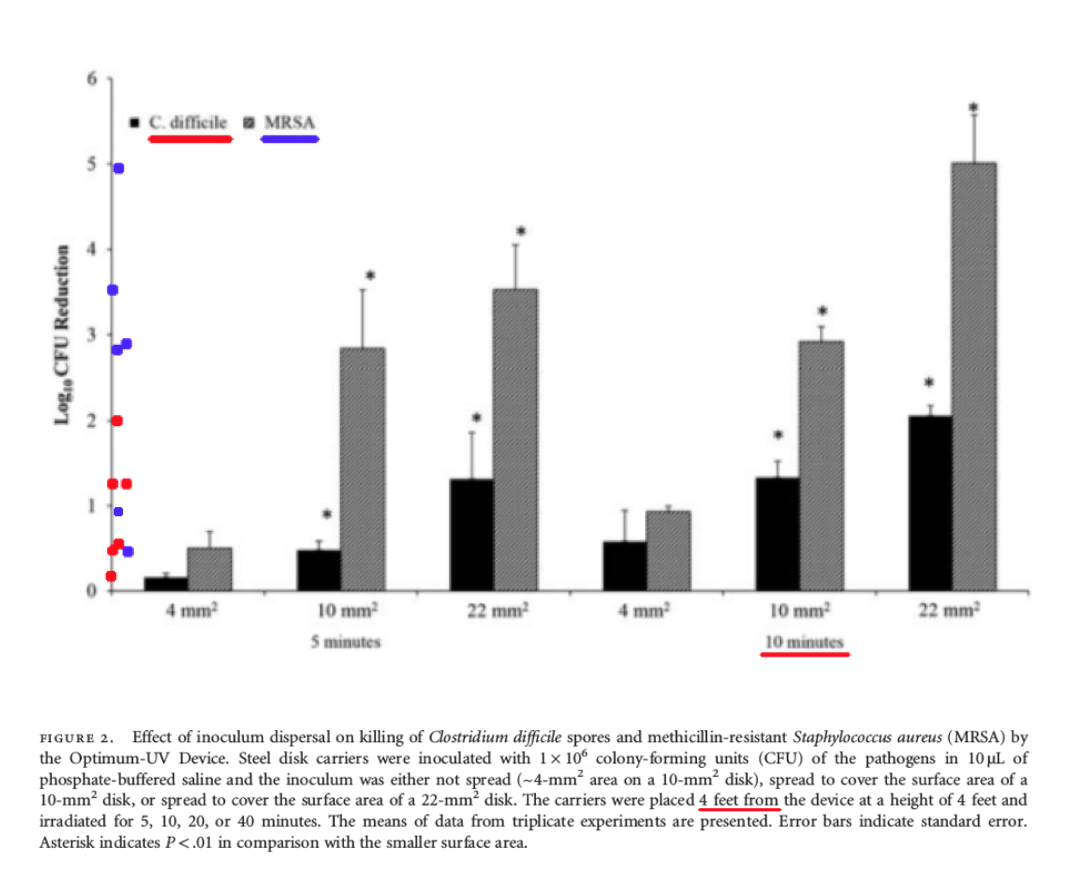
Comments – Figure 2: The data shown above in Figure 2 is important, because it shows the Log Reduction data at four (4) feet after ten (10) minutes of UV-C exposure, for bacteria that were spread over different sized disks. The Log Reduction data only ranged from about (0.6 - 2.0) for C. difficile spores.
Per Federal standards, when a test surface is contaminated with 1,000,000 bacteria spores, and a Log Reduction of about 0.6 Log to 2.0 Log is shown for C. difficile spores, between about 10,000 to 100,000+ C. difficile survivors will remain! This is NOT disinfection, decontamination, or sterilization. OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
Also in Figure 2, the Log Reduction data ranged from about (1.0 – 5.0) for the vegetative bacteria (non-spore) MRSA (Staphylococcus aureus), at four (4) feet after ten (10) minutes of UV-C exposure.
Per Federal standards, when a test surface is contaminated with 1,000,000 bacteria, and a Log Reduction of about 1.0 Log to 5.0 Log is obtained for MRSA (Staphylococcus aureus), that means there will still be between about 10 to 100,000 MRSA survivors remaining that can grow their population exponentially and infect people. This is NOT disinfection, decontamination, or sterilization. OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
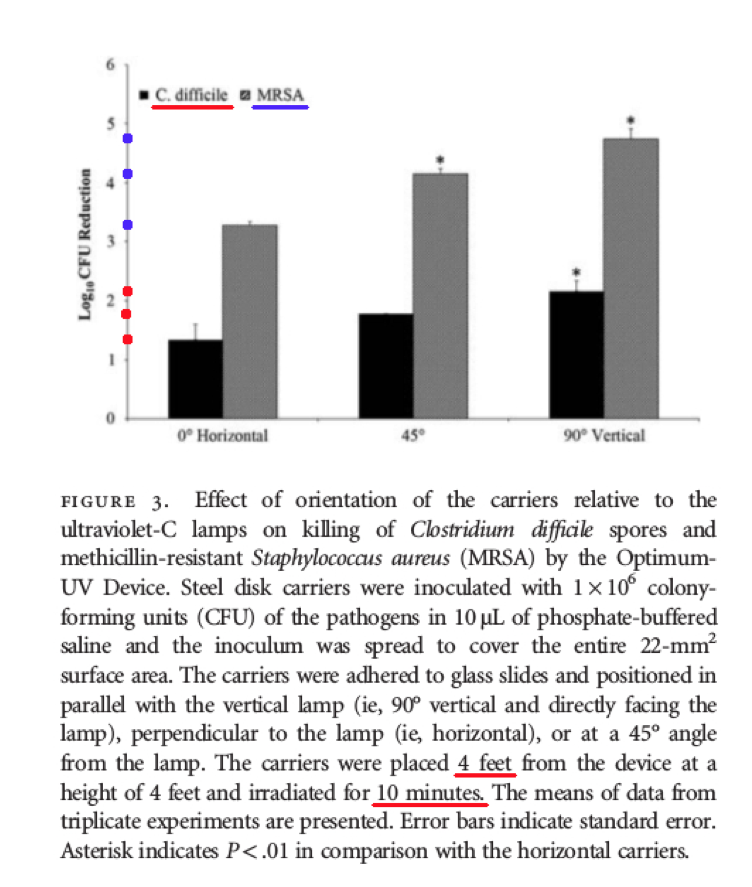
Comments – Figure 3: As shown below in Figure 3, the test media is exposed at four (4) feet for ten (10) minutes at different orientations to the UV-C light including at: zero (0) degree horizontal orientation, forty-five (45) degree orientation, and ninety (90) degree vertical orientation. The test results show Log Reduction data that ranged from only about (1.3 - 2.2) for C. difficile spores depending on the test orientation. The test results also showed Log Reduction data that ranged from only about (3.3 – 4.8) for MRSA (Staphylococcus aureus) depending on the test orientation.
When a test surface is contaminated with 1,000,000 bacteria spores, and a Log Reduction of about 1.3 Log to 2.2 Log is shown for C. difficile spores, that means there will still be between about 1,000 to 10,000+ C. difficile survivors remaining. Pathogenic bio-burden is a health risk. When a Log Reduction of about 3.3 Log to 4.8 Log is obtained for MRSA, that means there will still be between about 10 to 100+ MRSA survivors remaining that can exponentially increase their population. This is NOT disinfection, decontamination, or sterilization, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
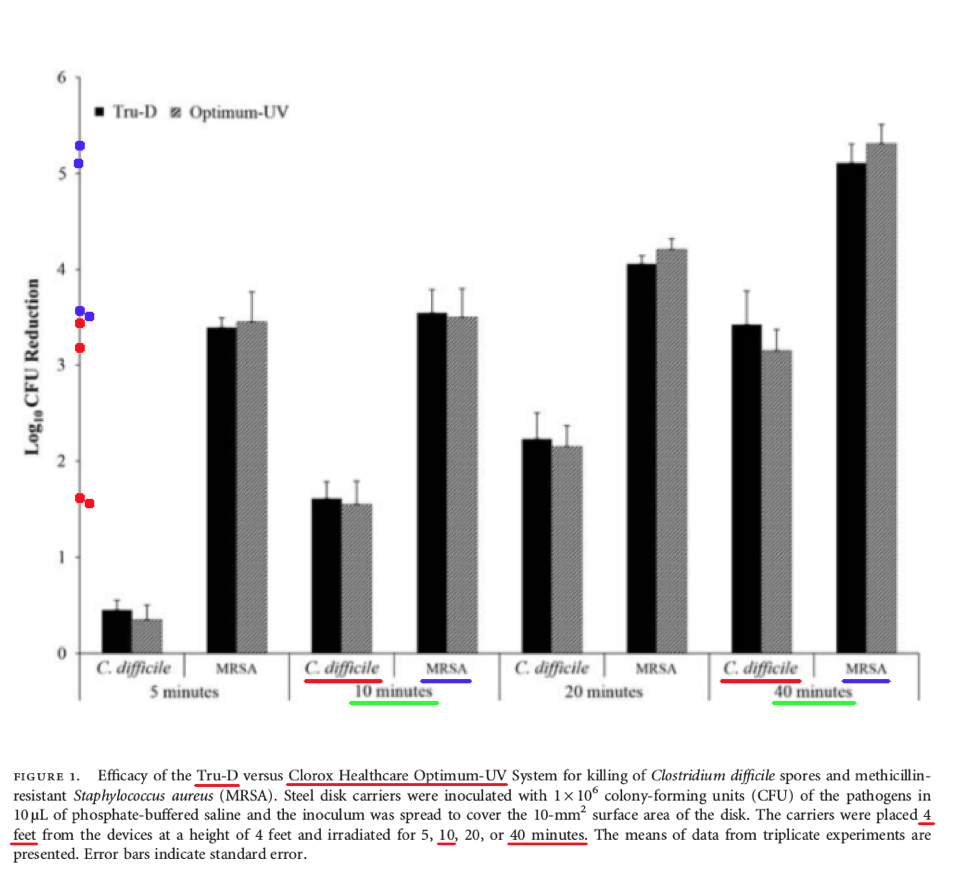
Comments – Figure 1: The data shown above in Figure 1 is important, because it shows the Log Reduction data at four (4) feet after ten (10) minutes, and also forty (40) minutes, of UV-C exposure, for the Tru-D UV-C product, and the Clorox Optimum UV-C product, for both MRSA bacteria and C. difficile spores.
The Log Reduction for C. difficile spores was about 1.7 Log for Tru-D UV-C, and 1.6 Log for Clorox UV-C, after ten (10) minutes of treatment.
Per Federal standards, when a test surface is contaminated with 1,000,000 bacteria spores, a Log Reduction of about 1.7 Log with Tru-D means there will be more than 10,000 C. difficile survivors remaining that can infect people, and a Log Reduction of about 1.6 Log with Clorox Optimum UV-C means there will also be more than 10,000 C. difficile spores remaining that can infect people. This is NOT disinfection, decontamination, or sterilization, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
The Log Reduction for C. difficile spores was about 3.4 Log for Tru-D UV-C, and 3.2 Log for Clorox UV-C, after forty (40) minutes of treatment.
Per Federal standards, when a test surface is contaminated with 1,000,000 bacteria spores, a Log Reduction of about 3.4 Log with Tru-D means there will be more than 100+ C. difficile survivors remaining. A Log Reduction of about 3.2 Log with Clorox Optimum UV-C means there will also be more than 100+ C. difficile spores remaining. This is NOT disinfection, decontamination, or sterilization, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
Conclusion: First, this study demonstrates that even after 40 minutes, both Tru-D's UV-C product, and Clorox's Optimum UV-C product, were still NOT able to reach a 6.0 Log performance level for either C. difficile or MRSA, and neither of these products can claim disinfection, hospital disinfection, or sterilization, per Federal regulations. (2)(3)
This study also reinforces the previously reported research data that UV-C light surface treatment is adversely impacted by not only the exposure time to the UV-C light source, but also the orientation or angles of the surfaces to the UV light source.
More importantly, per the United States EPA, these independent data show that UV-C room treatment systems do NOT meet the legal definitions for disinfection, hospital disinfection, sterilization, or as a sporicidal against C. difficle, per Federal regulations. (2)(3)
3) William Rutala, PhD, MPH, and David Weber, MD, MPH et al.: "Room Decontamination with UV Radiation", Infection Control & Hospital Epidemiology, October 2010, Vol. 31, No. 10. (7)
"The efficacy of UV irradiation is a function of many different location and operational factors, such as intensity, exposure time, lamp placement, and air movement patterns."
“In our test room, the effectiveness of UV-C radiation in reducing the counts of vegetative bacteria on surfaces was more than 99.9% in approximately 15 minutes, and the reduction in C. difficile spores was 99.8% within 50 minutes.”
Comment: According to Federal regulations, this is NOT disinfection, decontamination, or sterilization, that requires a 6.0 Log reduction or Percent Reduction of 99.9999%, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3) The 99.8% and 99.9% reported percent reductions only equates to a Log Reduction of about 3.0 Log, leaving viable organisms.
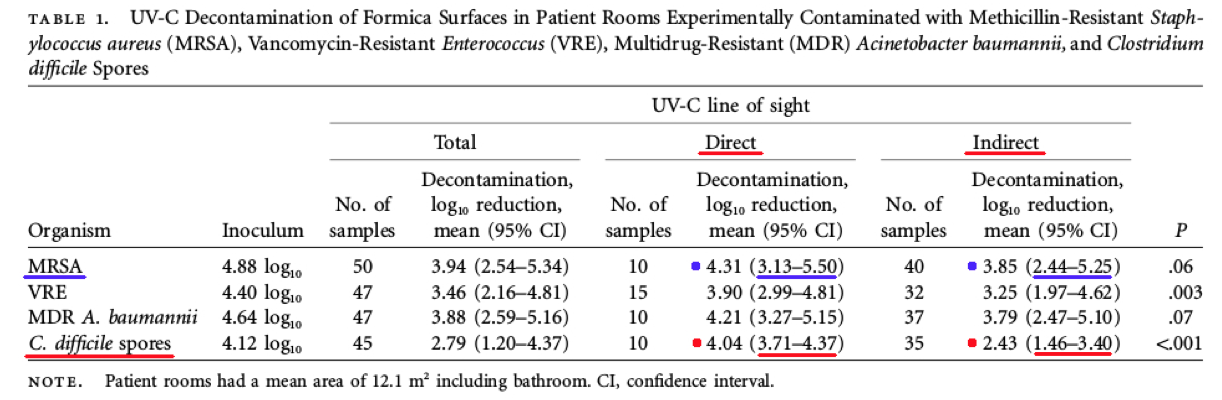
Comments – Table 1: The C. difficile spore data in Table 1 above shows a Log Reduction range of (3.71 to 4.37) for direct UV-C light exposure, and (1.46 to 3.40) for indirect UV-C light exposure.
If a test surface is contaminated with 1,000,000 bacteria, per Federal standards, and a Log Reduction of about 3.71 Log to 4.37 Log is achieved for C. difficile spores with exposure to direct UV-C light, that means there will still be between about 10 to 100+ C. difficile spore survivors remaining on surfaces. This is NOT disinfection, decontamination, or sterilization, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
Also, if a test surface is contaminated with 1,000,000 bacteria, per Federal standards, and a Log Reduction of about 1.46 Log to 3.40 Log is achieved for C. difficile spores with exposure to indirect UV-C light, that means there will still be between about 100 to 10,000+ C. difficile spore survivors remaining on surfaces. This is NOT disinfection, decontamination, or sterilization, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
The MRSA (Staphylococcus aureus) data in Table 1 above shows a Log Reduction range of (3.13 to 5.50) for direct UV-C light exposure, and (2.44 to 5.25) for indirect UV-C light exposure.
If a test surface is contaminated with 1,000,000 bacteria, per Federal standards, and a Log Reduction of about 3.13 Log to 5.50 Log is achieved for MRSA with exposure to direct UV-C light, that means there will still be between about 1 to 100+ MRSA survivors remaining that can exponentially increase their population and infect a person. This is NOT disinfection, decontamination, or sterilization, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
If a test surface is contaminated with 1,000,000 bacteria, per Federal standards, and a Log Reduction of about 2.44 Log to 5.25 Log is achieved for MRSA with exposure to indirect UV-C light, that means there will still be between about 1 to 1,000+ MRSA survivors remaining that can exponentially increase their population. This is NOT disinfection, decontamination, or sterilization, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
Conclusion: This study reinforces the current research data that UV-C light treatment process is adversely impacted by shadowed surfaces. More important, per the United States EPA and Federal regulations, this data shows that UV-C room treatment system results and claims do NOT meet the legal definitions for disinfection, hospital disinfection, sterilization, or as a sporicidal against C. difficle. (2)(3)
4) John M. Boyce, MD, et al.: “Impact of Room Location on UV-C Irradiance and UV-C Dosage and Antimicrobial Effect Delivered By A Mobile UV-C Light Device”, Infection Control & Hospital Epidemiology, June 2016, Vol. 37, NO. 6. (5)
“UV-C irradiance, UV-C dosage, and antimicrobial effect achieved in patient rooms varied significantly, depending on the location and orientation of surfaces relative to the UV-C device.”
“With 15-minute cycles, counts of MRSA on disks were reduced by 3 to >4 log10 and VRE by 1–4 log10 at varying distances and orientations relative to the UV-C device (Table 2). Log10 reductions of C. difficile were highest (2 to >4 log10 ) when disks were facing the device at a distance of 1.3 m and were lowest (0–1 log10 ) when disks were in a shaded area 3.3 m from the device (Table 2).” (emphasis added)
Comments - Referring below, to Table 2 and the data column for a 15 minute cycle (far right), the UV-C device was NOT able to achieve even close to a 6 Log Reduction for disinfection, in direct light at even 1.3 meters, for vegetative bacteria like MRSA, and VRE, as well as C. difficile spores. Instead, the UV-C product achieved a maximum performance of only around a >4.0 Log Reduction. This is NOT disinfection, decontamination, or sterilization, as defined by the EPA. (2)(3)
However, more concerning was how the UV light performance was significantly degraded at even a short distance (1.3 meters) in situations where the MRSA, and VRE, as well as C. difficile spores, were exposed to the UV light at a zero (0) degree angle for a 15 minute cycle, providing a low Log Reduction range of only (3.0 – 4.0) for VRE, a low Log Reduction of only around >4.0 Log for MRSA, and a low Log Reduction range of only (2.0 – 4.0) for C. difficile!
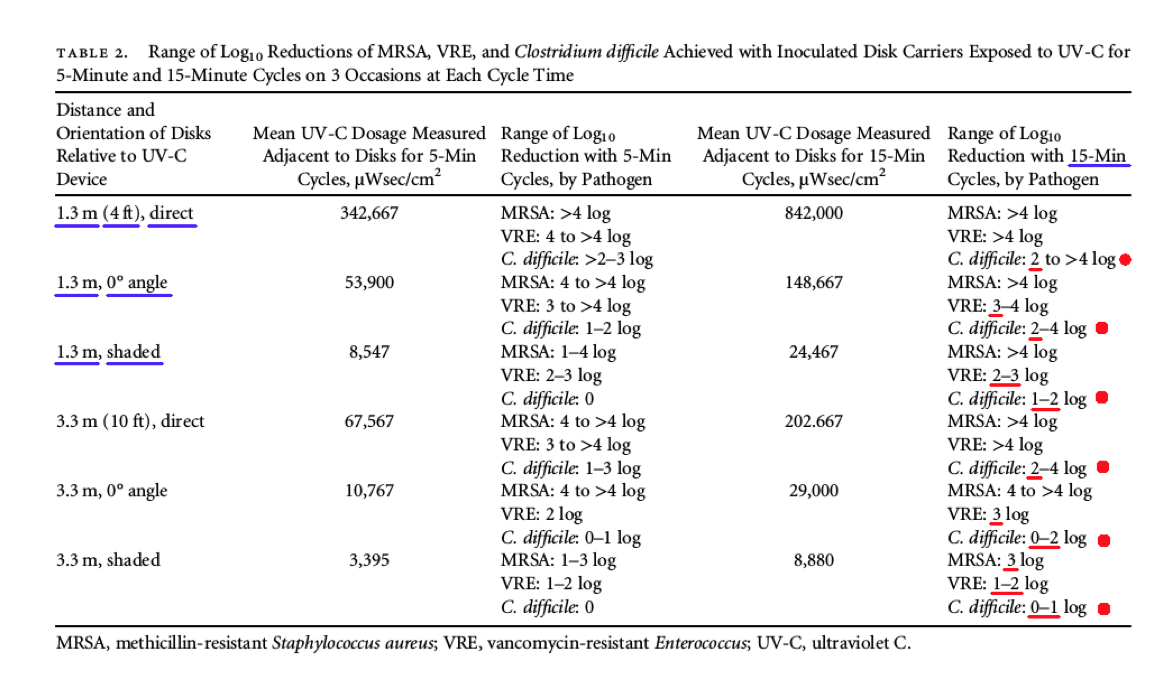
* NOTE: The data in Table 2 represents the range of “Log Reduction” data for MRSA, VRE, and Clostridium difficile (C. Difficile) spores, where the innoculated disks were placed at six (6) different locations with respect to the UV-C device: direct light, angled light at zero (0) degrees, and shaded, at two (2) different distances: 1.3 meters (4 feet) and 3.3 meters (10 feet).
Even more alarming regarding Table 2 above, is how the UV light performance was significantly degraded at even a short distance (1.3 meters) in situations where the bacteria and spores were shaded from the UV light for a 15 minute cycle, providing an even lower Log Reduction range of only (2.0 – 3.0) for VRE, a low Log Reduction of only around >4.0 Log for MRSA, and an extremely low Log Reduction range of only (1.0 – 2.0) for C. difficile.
Finally, the UV light performance was very degraded at ten (10) feet or (3.3 meters) in situations where the bacteria and spores were shaded from the UV light providing an extremely low Log Reduction range of only (1.0 – 2.0) for VRE, a low Log Reduction of only around 3.0 Log for MRSA, and a shockingly low Log Reduction range of only (0 – 1.0) for C. difficile! When exposed to the UV light at a zero (0) degree angle, for a 15 minute cycle, only a shockingly low Log Reduction range of (0 – 2.0) was achieved for C. difficile.
However, Cadnum and Dr. Donskey et al. (2016) (6), show that even a 40 minute exposure time in the most favorable exposure orientation of facing the UV-C light (sold by Tru-D and Clorox), at only 1.22 meters, only provides a best case Log Reduction of about 5.3 Log for the vegetative bacteria (non-spore) MRSA (Staphylococcus aureus), and an even worse best case Log Reduction of only 3.3 Log for C. difficle spores. Obviously, after even 40 minutes of exposure, UV-C cannot meet the Federal standards for a 6.0 Log Reduction to claim Disinfection, and UV-C cannot meet the Federal Standards of “no growth” to claim efficacy for C. difficle spores.
Conclusion: The various data shown above in Table 2 and provided by Dr. Boyce et al. (2016), show that a UV-C light room treatment system is adversely impacted by surface angles, shadowing, and distance from the UV light source, and does NOT meet the legal definitions for disinfection, hospital disinfection, sterilization, or as a sporicidal against C. difficle, per Federal laws. (2)(3)
5) Michelle Nerandzic, and Curtis Donskey, MD et al.: "Evaluation of a Pulsed Xenon Ultraviolet Disinfection System for Reduction of Healthcare-Associated Pathogens in Hospital Rooms", Infection Control & Hospital Epidemiology, February 2015, Vol. 36 No 2. (4)
“As shown in Figure 3, the efficacy of PX-UV decreased as distance from the device increased. For each pathogen, significantly less reduction was achieved at 4 feet versus 6 inches and at 10 feet versus 4 feet (P < .05 for each comparison) .... At 10 feet from the device, the log 10 CFU reduction was less than 1 log 10 CFU/cm 2 for each pathogen." (emphasis added)
“The efficacy of PX-UV was dramatically reduced as the distance from the device was increased.” (emphasis added)
* Important Note: PX-UV = Pulsed UV product, sold by Xenex
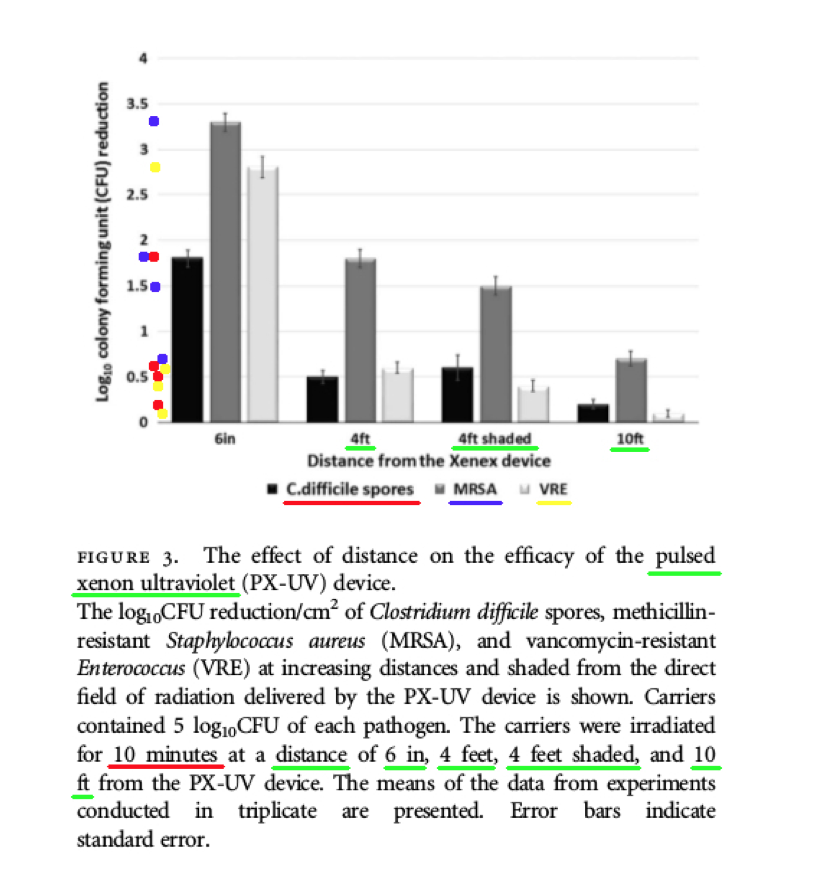
Comments – Figure 3: The data shown above in Figure 3 is important, because it shows the Log Reduction data after ten (10) minutes of PX-UV exposure, for MRSA and VRE bacteria, and C. difficile spores, at the following distances and conditions: four (4) feet, four (4) feet (and shaded), and ten (10) feet. The Log Reductions are as follows:
-------------------------------------------------------------------------------------------------
4 ft. 10 minutes C. difficile spores 0.5 Log Reduction (aprox.)
4 ft. 10 minutes (shaded) C. difficile spores 0.6 Log Reduction (aprox.)
10 ft. 10 minutes C. difficile spores 0.2 Log Reduction (aprox.)
-------------------------------------------------------------------------------------------------
4 ft. 10 minutes MRSA 1.8 Log Reduction (aprox.)
4 ft. 10 minutes (shaded) MRSA 1.5 Log Reduction (aprox.)
10 ft. 10 minutes MRSA 0.7 Log Reduction (aprox.)
-------------------------------------------------------------------------------------------------
4 ft. 10 minutes VRE 0.6 Log Reduction (aprox.)
4 ft. 10 minutes (shaded) VRE 0.4 Log Reduction (aprox.)
10 ft. 10 minutes VRE 0.1 Log Reduction (aprox.)
-------------------------------------------------------------------------------------------------
According to these data from Figure 3, the Xenex PX-UV light provided extremely low Log Reductions, and NONE of these Log Reduction values (C-diff. Spores and MRSA) are even close to meeting the Federal requirements to claim: disinfection, hospital disinfection, or sterilization, per the following EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3) The Xenex PX-UV light was NOT even able to achieve a Log Reduction anywhere close to the 5.0 Log amount of inoculum applied to the test slides.
Also, the Figure 3 data shows how drastically diminished the Log Reduction values were, when observed at a distance of ten (10) feet from the UV light source. The highest Log Reduction recorded was for MRSA, with a Log Reduction of only 0.7 Log, which is no where even close to the 5.0 Log amount of inoculum applied to the test slides, and certainly does NOT meet the EPA standards.
Comments – Figure 4: The data shown below in Figure 4 shows the low Log Reduction performance for both the Xenex PX-UV light product, and the continuous mercury UV-C light product.
-----------------------------------------------------------------------------------------------------------------------
4 ft. 10 minutes C. difficile spores 1.0 Log Reduction (aprox.) - UV-C
4 ft. 10 minutes C. difficile spores 0.5 Log Reduction (aprox.) - Xenex, PX-UV
-----------------------------------------------------------------------------------------------------------------------
4 ft. 10 minutes MRSA 3.1 Log Reduction (aprox.) - UV-C
4 ft. 10 minutes MRSA 1.8 Log Reduction (aprox.) - Xenex, PX-UV
-----------------------------------------------------------------------------------------------------------------------
4 ft. 10 minutes VRE 3.6 Log Reduction (aprox.) - UV-C
4 ft. 10 minutes VRE 0.6 Log Reduction (aprox.) - Xenex, PX-UV
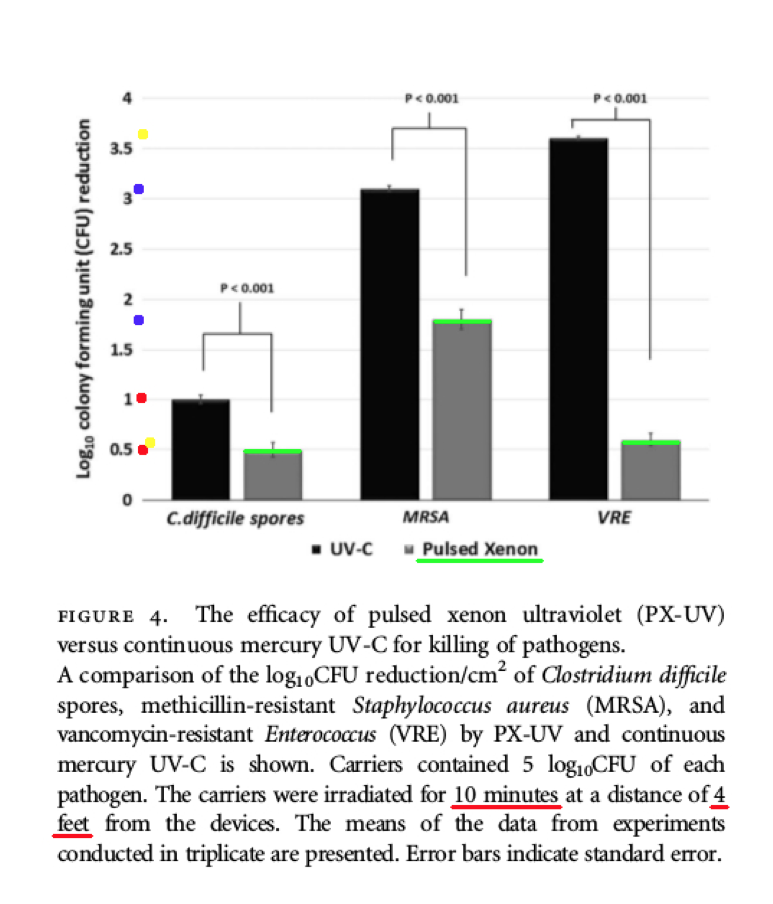
According to the data above from Figure 4, both the Xenex PX-UV light and the continuous mercury UV-C light product, failed to produce Log Reduction values (C-diff. spores and MRSA) that can satisfy the Federal requirements to claim: disinfection, hospital disinfection, or sterilization. OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
The data shown above in Figure 4 is important, because it shows the poor Log Reduction data at four (4) feet after ten (10) minutes of PX-UV and UV-C light exposure. The Log Reduction data was reported at an extremely low Log Reduction of 0.5 Log for C. difficile spores by the Xenex PX-UV product, and an extremely low Low Reduction of 1.0 Log for C. difficile spores by the continuous mercury UV-C light product.
If a test surface is contaminated with 1,000,000 bacteria spores, and a Log Reduction of only 0.5 Log is obtained by Xenex PX-UV for C. difficile spores, that means more than 100,000+ C. difficile spore survivors will remain on the treated surfaces! This is NOT disinfection, decontamination, or sterilization, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
In addition, the Figure 4 data shows that when a test surface is contaminated with 1,000,000 bacteria spores, and a Log Reduction of only 1.0 Log for UV-C light is achieved for C. difficile spores, that means 100,000 C. difficile spores will survive on the surface! This is NOT disinfection, decontamination, or sterilization, per the EPA standards: OCSPP 810.2200 (3), OCSPP 810.2200 (5) & (6), and OCSPPP 810.2100 (d)(2) and (g). (2)(3)
Conclusion: This study reinforces the previously reported research data that both the Xenex PX-UV light product, and the continuous mercury UV-C light product, are adversely impacted by the distance of the treated surfaces to the UV light source, and do NOT meet the EPA performance requirements for disinfection, hospital disinfection, sterilization, or as a sporicidal against C. difficle. (2)(3) Any claim of being able to “disinfect an entire room” flies in the face of this data.
6) Louis Stokes VA Hospital, Cleveland, OH, 2017, FedBizOpps, Solicitation Number: VA250-17-Q-0774
“The number of C-Diff rooms has increased, despite current sanitation procedures …. The Louis Stokes Cleveland VA Medical Center currently utilizes the Tru-D Smart UVC Part Number: 0367AOLF, but we are still not getting the desired results and the level of disinfection expected to especially hard to reach areas.” (emphasis added) (1)
7) Irene Louh, MD, PhD, and Henry Ting, MD, et al.: “Clostridium Difficile Infection in Acute Care Hospitals: Systematic Review and Best Practices for Prevention”, Infection Control & Hospital Epidemiology, April 2017, Vol. 38, NO. 4. (10)
“Terminal cleaning with UV light in addition to bleach cleaning had uncertain efficacy.” (emphasis added)
“Haas et. al. instituted pulsed UV treatment in addition to terminal bleach disinfection in a large urban hospital, with minimal incremental reduction in CDI rates.” (emphasis added)
8) U.S. CDC - Clinical Alert to U.S. Healthcare Facilities - June 2016, U.S. Centers For Disease Control & Prevention, “Global Emergence Of Invasive Infections Caused By The Multidrug-Resistant Yeast Candida auris”, June 24, 2016 (last updated: 2017). (9)
https://www.cdc.gov/fungal/diseases/candidiasis/candida-auris-alert.html
“The Centers for Disease Control and Prevention (CDC) has received reports from international healthcare facilities that Candida auris, an emerging multidrug-resistant (MDR) yeast, is causing invasive healthcare-associated infections with high mortality. Some strains of C. auris have elevated minimum inhibitory concentrations (MICs) to the three major classes of antifungals, severely limiting treatment options." (emphasis added)
"Environmental Cleaning – Anecdotal reports have suggested that C. auris may persist in the environment. Healthcare facilities who have patients with C. auris infection or colonization should ensure thorough daily and terminal cleaning and disinfection of these patient’s rooms using an EPA-registered hospital grade disinfectant with a fungal claim." (emphasis added)
Comment: The situation with C. auris, is a serious threat to human safety, and very specific standards are currently specified by the CDC, to address C. auris. UV-C and PX-UV are NOT mentioned by the CDC as an approved treatment to address the C. auris threat. Only disinfectants that can meet the United States EPA standards for hospital disinfection (OCSPP 810.2200 (5) & (6)), and fungal claims (OCSPP 810.2200 (9)(e)), are approved by the CDC to counter C. auris.
VI. Log Reduction Reference
Log Reduction Number of cfu's Percent Reduction
0 log (Log 0) 1,000,000 0%
1 log (Log 1) 100,000 90%
2 log (Log 2) 10,000 99%
3 log (Log 3) 1,000 99.9%
4 log (Log 4) 100 99.99%
5 log (Log 5) 10 99.999%
6 log (Log 6) 1 99.9999%
VII. UNITED STATES FEDERAL DEFINITIONS FOR “DISINFECTANTS”, “HOSPITAL DISINFECTANTS”, AND “STERILANTS”

a) US Legal Definition for “General Disinfection / Broad Spectrum Efficacy”
Reference: OCSPP 810.2200 (3)
DEFINITION: General or broad spectrum efficacy products - When a disinfectant is represented in labeling as having efficacy against both Gram-negative and Gram-positive bacteria, the product is considered a “general or broad spectrum” disinfectant.
According to the United States Environmental Protection Agency (EPA), “Disinfection” is defined as set forth in EPA Product Performance Test Guidelines, OCSPP 810.2200.
The test microorganisms are:
1) Effective against both Gram-negative and Gram-positive bacteria.
2) Staphylococcus aureus (S. aureus)(ATCC 6538) for effectiveness against Gram-positive bacteria.
3) Salmonella enterica (ATCC 10708) (S. enterica) for effectiveness against Gram-negative bacteria.
The test criteria states:
"Evaluation of confirmatory general or broad spectrum disinfectant success. The product should kill all the test microorganisms on all carriers in ≤ten minutes. In addition, per the 2009 AOAC revisions for the Use-Dilution Method, the mean log density for S. aureus is to be at least 6.0 (corresponding to a geometric mean density of 1.0 x 10^6 ); a mean log density <6.0 invalidates the test. For the Hard Surface Carrier Test, the dried carrier counts should be 0.5 –2.0 x 10^6 for Salmonella enterica and 1 – 5 x 10^6 for Staphylococcus aureus." (emphasis added) (2)
* Summary: To meet the definition of “General Disinfection” a 6 log kill has to be obtained for both “Staph” and “Salmonella” in less than 10 minutes.
b) US Legal Definition for “Hospital Disinfection”
Reference: OCSPP 810.2200 (5) & (6)
The EPA has a specific category established for the hospital and healthcare markets. For these markets, the following efficacy is required to meet the definition of disinfection as set forth in EPA Product Performance Test Guidelines, OCSPP 810.2200.
The test microorganisms are:
1) Effective against both Gram-negative and Gram-positive bacteria.
2) Staphylococcus aureus (S. aureus)(ATCC 6538) for effectiveness against Gram-positive bacteria.
3) Pseudomonas aeruginosa (P. aeruginosa)(ATCC 15442) for effectiveness against Gram-negative bacteria.
The test criteria states:
“Evaluation of confirmatory hospital or healthcare disinfectant success. The product should kill all the test microorganisms on all carriers in ≤ten minutes. In addition, per the 2009 AOAC revisions for the Use-Dilution Method, the mean log density for S. aureus and P. aeruginosa is to be at least 6.0 (corresponding to a geometric mean density of 1.0 x 10^6); a mean log density <6.0 invalidates the test. For the Hard Surface Carrier Test, the dried carrier counts should be 1 –5 x 10^6 for both Staphylococcus aureus and Pseudomonas aeruginosa.” (emphasis added) (2)
* Summary: To meet the definition of “Hospital Disinfection” a 6 log kill has to be obtained for both “Staph” and “Pseudomonas” in less than 10 minutes.
c) US Legal Definition for “Disinfectants With Fungicidal Claims”
Reference: OCSPP 810.2200 (9)(e)
The test microorganism is:
1) Trichophyton mentagrophytes (T.mentagrophytes)(ATCC 9533)
Two samples representing two different batches of the product should be evaluated for efficacy against Trichophyton mentagrophytes (T. mentagrophytes)(ATCC 9533). The inoculum employed should provide a concentration of ≥5 x 10^6 conidia/mL.
Evaluation of fungicidal success. For the AOAC International Fungicidal Activity of Disinfectants test, all fungal spores at 10 and 15 minutes should be killed to support a 10 minute exposure time. For the AOAC International Use-Dilution Methods, all fungal spores on all 10 carriers should be killed in ≤ten minutes. (emphasis added) (2)
d) US Legal Definition for “Sterilant w/ Clostridium difficile Claims”
Reference: OCSPPP 810.2100 (d)(2) and (g)
General Liquid Sterilants Claims - Mandated Log Reductions:
5-6 Log reduction minimum for BOTH Bacillus subtilis (B. subtilis) spores and Clostridium sporogenes (C. sporogenes) spores, AND must reach at least 6 Log reduction minimum for Clostridium difficile (C. difficile) spores, to be classed as liquid Sterilant w/ Clostridium difficile (C. difficile) Claims. Kill everything, no growth, on ALL slides in less than XX minutes (time not specified).
The test microorganisms are:
1) Effective against: (B. subtilis) and (C. sporogenes) and (C. difficile)
2) Clostridium difficile (C. difficile) (ATCC 700792), (ATCC 43598) or (ATCC 43599)
3) Bacillus subtilis (B. subtilis) (ATCC 19659)
4) Clostridium sporogenes (C. sporogenes) (ATCC 3584)
Evaluation of sterilant success. The inoculum employed should provide a count of 1 x 10^5 – 1 x 10^6 spores per carrier. The product should kill the test spores on all 120 carriers without any failures (e.g., growth of test organism after carrier treatment constitutes failure). (3)
VIII. REFERENCES
1) Louis Stokes Cleveland, 2017, FedBizOpps Solicitation Number: VA250-17-Q-0774, FedBizOpps
2) United States Govt., https://www.regulations.gov/document?D=EPA-HQ-OPPT-2009-0150-0021
3) United States Govt., https://www.regulations.gov/document?D=EPA-HQ-OPPT-2009-0150-0020
4) Michelle Nerandzic, and Curtis Donskey, MD et al.: "Evaluation of a Pulsed Xenon Ultraviolet Disinfection System for Reduction of Healthcare-Associated Pathogens in Hospital Rooms", Infection Control & Hospital Epidemiology, February 2015, Vol. 36 No 2.
5) John M. Boyce, MD, et al.: “Impact of Room Location on UV-C Irradiance and UV-C Dosage and Antimicrobial Effect Delivered By A Mobile UV-C Light Device”, Infection Control & Hospital Epidemiology, June 2016, Vol. 37, NO. 6.
6) Jennifer Cadnum, and Curtis Donskey, MD, et al.: "Effect of Variation in Test methods on Performance of Ultraviolet-C Radiation Room Decontamination", Infection Control & Hospital Epidemiology, November 2016.
7) William Rutala, PhD, MPH, and David Weber, MD, MPH et al.: "Room Decontamination with UV Radiation", Infection Control & Hospital Epidemiology, October 2010, Vol. 31, No. 10.
8) Michelle Nerandzic, and Curtis Donskey, MD et al.: "Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms", BioMedCentral, BMC Infectious Diseases, 2010, 10:197.
9) U.S. CDC - Clinical Alert to U.S. Healthcare Facilities - June 2016, U.S. Centers For Disease Control & Prevention, “Global Emergence Of Invasive Infections Caused By The Multidrug-Resistant Yeast Candida auris”, June 24, 2016 (last updated: 2017).
10) Irene Louh, MD, PhD, and Henry Ting, MD, et al.: “Clostridium Difficile Infection in Acute Care Hospitals: Systematic Review and Best Practices for Prevention”, Infection Control & Hospital Epidemiology, April 2017, Vol. 38, NO. 4.
11) Xenex - https://www.xenex.com/about-xenex
12) Tru-D - http://tru-d.com/benefits/
13) Surfacide - http://www.surfacide.com/
14) Sreelatha Koganti, MD, and Curtis Donskey, MD - "Evalution of Hospital Floors as a Potential Source of Pathogen Dissemination Using a Nonpathogenic Virus as a Surrogate Marker", Infection Control & Hospital Epidemiology, November 2016, Vol. 37, No. 11.

