Why Altapure? Unmatched Rapid Spore Kill:
“In all the testing that I have done over the years at Raven (An independent USA Laboratory, FDA registered, ISO Certified), I have never seen any method kill both G. stearothermophilus and B. atrophaeus in less than fifteen minutes and yours was showing all killed in less than a minute. I was floored.”
Deb Dwyer
Director of Operations, Mesa Laboratories Inc. (Raven Biological Laboratories)
www.mesalabs.com
Altapure Process: Safe - Rapid - Efficacious
- 6 Log Kill: Spores * Viruses * Bacteria ™
- Immediate Room Turn-Around ™ (under 45 minutes - common patient room)
This is what sets Altapure apart from all other “no touch” high-level disinfection / surface decontamination systems that are currently available.
Altapure believes that “scientific facts” and not glossy marketing brochures should be the driver for any purchasing decisions. The health of our friends and loved ones are counting on it.
Since 2004, Altapure continues to work to develop the best high-level disinfection / surface decontamination system in the global market place.
Altapure, LLC has worked jointly with Harris Corporation (NYSE: HRS), a top-tier global aerospace, defense, and information solutions company, to develop a new patented technology, to deploy an extremely thick cloud of gas-like sub-micron aerosol, that treats, high-level disinfects, and decontaminates the surfaces in targeted room(s) and space(s).
Altapure offers:
- Pandemic Performance - Ability to fully treat and process 24 patient rooms in 24 hours.
- Rapid - Patient room entry (Less than 50 minutes - common patient room).
- Immediate occupation of room after treatment cycle ends.
- 2.3 – 2.6 D-Value (Time in minutes required to kill 90% of organisms being tested).
- No Shadowing on ANY exposed surfaces.
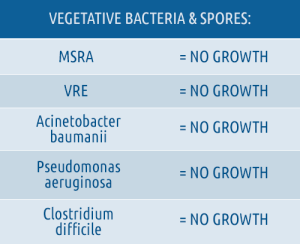
- Clinically proven “No Growth” on ALL treated room surfaces for: Bacterial Spores, Viruses, & Vegetative Bacteria.
- Gas-like Diffusion & Performance (sub-micron aerosol).
- "Extremely thin film” deposition via sub-micron droplet performance.
- Multi Room Capable - Able to treat connected areas & rooms & around corners.
- Gas-Like Interaction / 3-D Contact With Complex Surface Geometries.
- Extremely large area capable (8,000+ cubic feet).
- Scalable with multiple machines (single point controlled).
- Complete Coverage Accuracy.
- Complete Coverage Reproducibility.
- Fully automated & remotely controlled process.
- “ No Growth ” efficacy despite distance.
- Extremely quiet operation.
- Closed-Loop Process Control ™.
- Wireless control and data reporting to PC.
- Easily moved and setup by only one (1) individual.
Extremely Low Hydrogen Peroxide Content
Yes! Altapure has an extremely low Hydrogen Peroxide content, and it is:
- Only 0.88 % H2O2 and 0.18 % PAA (Yes, the hydrogen peroxide is that low).
- Green / Environmentally friendly process.
- Broad material compatibility & gentle on equipment.
- Completely Biodegradable. (breaks down into water, oxygen, and vinegar vapor).
- Pharmaceutical grade ingredients.
- Residue-free.
Altapure uses a very small percentage of ingredients, unlike our competitors who use vaporized hydrogen peroxide (VHP), and apply a minimum of 35% Hydrogen Peroxide / VHP, onto surfaces in rooms, or even other competitors that try to aerosolize 7.5% Hydrogen Peroxide, which is still a very high percentage of Hydrogen Peroxide.
For more information about Altapure and its process, please visit our Technology Section.
The Clostridium difficile (C. difficile / CDF / C-Diff.) Dilemma
Clostridium difficile, a spore forming bacteria, has redefined the “no touch” high-level disinfection industry, as inadequate technologies continue to lose favor and drop out of the marketplace, and are replaced with better methods to prevent hospital acquired infections (HAI's).
The problem with C. difficile is that patients are accidentally ingesting the spores of this bacteria while they are in hospitals, nursing homes, or other health care facilities. C. difficile is responsible for almost half a million infections and is associated with approximately 29,000 deaths in 2011. (Funded by the Centers for Disease Control and Prevention.).
Resource: New England Journal of Medicine
Resource: Center for Disease Control
** This is a very serious problem. Products and technologies that are weak in their surface treatment, and FAIL TO ELIMINATE ALL OF THE SPORES in the health care setting are only putting people's lives at serious risk, and creating unwarranted confidence, for those that are relying on these technologies to save lives. The importance of this issue was demonstrated by Dr. Curtis Donskey when a simulated pathogen was shown to be easily moved from room to room in a hospital (2016) (94).
Click here for study: https://pubmed.ncbi.nlm.nih.gov/27523489/
In light of the fact that many people die within 30 days of acquiring a C. difficile infection, technologies that cannot eliminate all of the C. difficile spores in the health care environment are inadequate. Furthermore, the failure to effectively challenge (placing pathogens in various locations within a room) various "disinfection" equipment, has also contributed to the myth that all no-touch disinfection technologies are equally effective.
Failure to eradicate C. difficile / C-Diff by competitive technologies results from:
- Fading power & efficacy as a function of distance.
- Incomplete surface coverage in treated space.
- Shadowing caused by objects in the room obstructing UV light (line of sight issues).
- Weak chemistry & weak spore kill activity.
- Large D-Values, if the treatment time is cut short.
- Absence of feedback / testing for process validation.
Altapure's technology does NOT have these limitations since it was specifically designed to completely eradicate C. difficile spore contamination on all exposed surfaces within the clinical environment, creating a new “No Growth Standard ”. Altapure provides its customers and their patients with the confidence and certainty that the treated environment is completely devoid of all surface contamination presented by: spores, viruses, and vegetative bacteria such as:
Test data obtained in a study with Dr. Denis Maki at the University of Wisconsin showed a “ Total Kill " of test challenges, including spores, on the following surfaces:
- The bed headboard
- Curtain
- Handrail
- Bedside table
- Mattress
- Windowsill
- Cabinet door
- Bedside dresser
- Toilet seat
- Sink basin
- Floor
- Doorknob
3-Dimensional Spore Kill
Unlike certain treatment modalities such as UV light, Altapure can treat 3-dimensionally like a gas, and it can reach hard to see surfaces, like under a handrail or behind a door knob, where surface contamination like C. difficile spores can hide.
Quick Facts of the AP-4 System with an EPA Approved PAA Agent:
6 Log + Kill: C. difficile spores, VRE, CRE, MRSA, viruses
Rapid: Less than 50 Minutes (Entry to Exit)
Low %: Only 0.88 % H2O2 & 0.18 % PAA
Gas-like Performance & Complete Large Area Coverage
Residue Free
Completely Biodegradable / Ends Green
Automated Process: tablet control with data reports
Non-Corrosive: safe for all electronics
100,000+ Hospital Deploys With No Equipment Damage